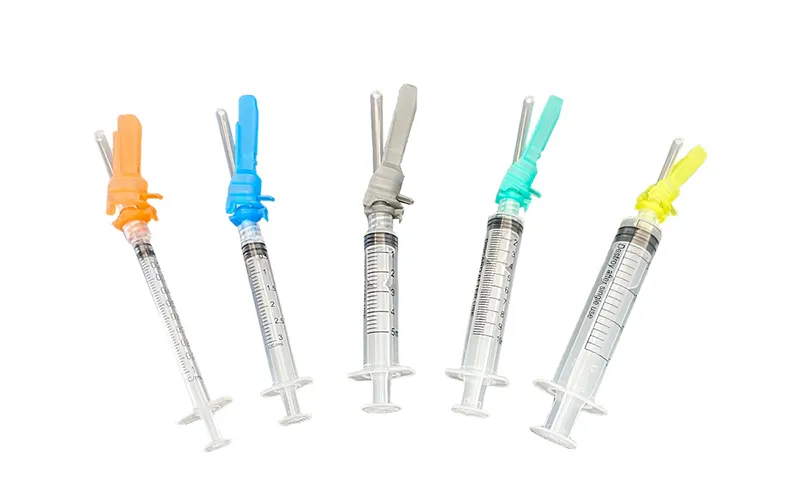
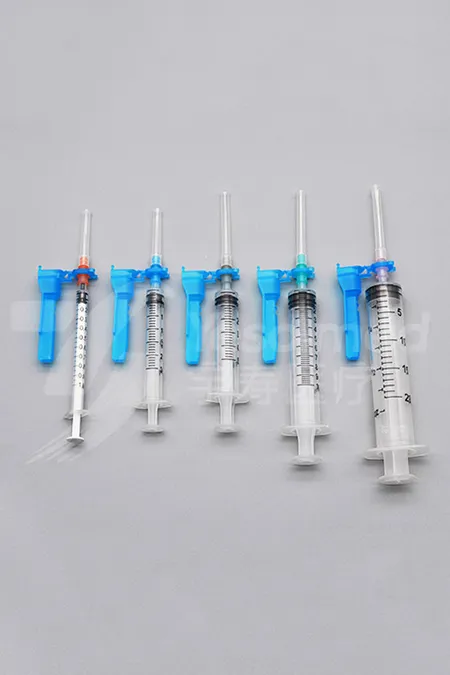
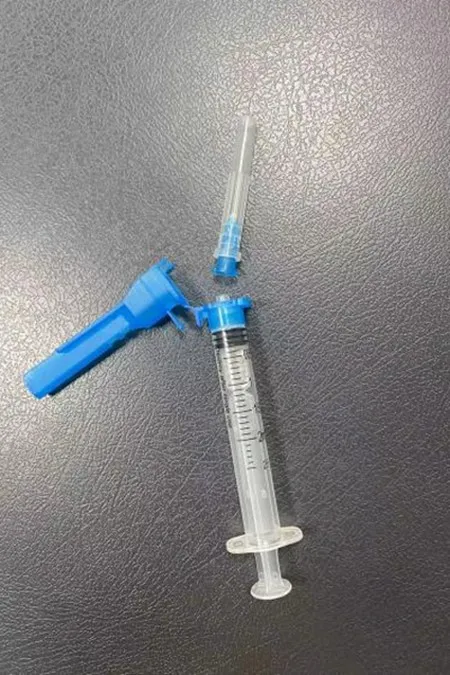
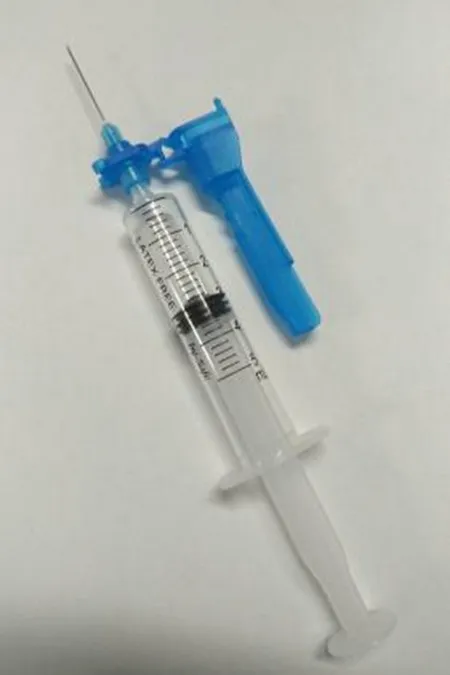
Safety Syringe
- Approval: US-FDA 510(k),EU-MDR and ISO13485
- Sterilization Method: ethylene oxide sterilization, non-toxic, non-pyrogenic
- Syringe Components: syringe barrel, plunger rod and stopper (piston)
- Syringe Volume: 1ml, 1.2ml, 2mL, 2.5mL, 3 ml, 5ml, 6ml, 10ml, 20ml, 30ml, 50ml, 60ml, and 100ml
- Needle Components: needle hub, needle shaft and pivoting shield
- Needle Gauge: 27G, 26G, 25G, 24G, 23G, 22G, 21G, 20G, 19G, and 18G
- US FDA 510 (k) No.: K053519
- EU MDR No.: G10 045084 0043 Rev.00
- EU ISO13485 No.: Q5 045084 0039 Rev.01